Fabulous Info About Is A Photon Bigger Than An Electron
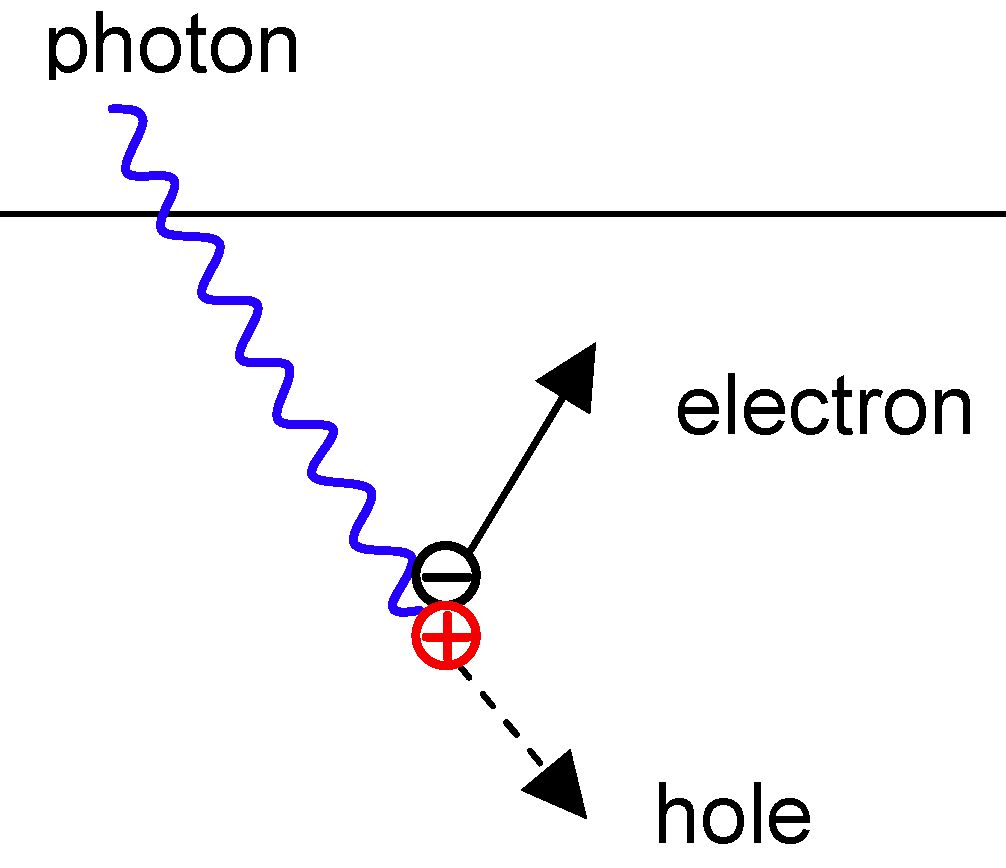
Is a Photon Bigger Than an Electron? A Question of Size, or Something Else Entirely?
1. What We Mean by "Size"
Alright, let's tackle this head-on. The question "Is a photon bigger than an electron?" sounds straightforward, but when you dive into the quantum realm, things get delightfully weird. When we usually think of "size," we picture something we can hold, measure with a ruler, and compare to other objects. Like saying a basketball is bigger than a tennis ball. But subatomic particles? They play by different rules.
Electrons and photons aren't like tiny marbles. They're more like fuzzy clouds of probability described by quantum mechanics. They exhibit wave-particle duality, meaning they act sometimes like particles (discrete packets of energy or mass) and sometimes like waves (spread out disturbances). So, asking about their "size" requires us to be a little more specific about what we actually mean by size. Are we talking about spatial extent? Probability distribution? Something else?
Think of it like asking if a musical note is "bigger" than a brushstroke. They're fundamentally different things, existing in different realms. A musical note has frequency, duration, and timbre; a brushstroke has width, color, and texture. You can't directly compare them in terms of simple size. Similarly, comparing the "size" of a photon and an electron is comparing apples and oranges... or maybe apples and abstract mathematical constructs!
So, before we can answer, we need to unpack the "size" question itself. Prepare for a journey into the strange and wonderful world of quantum mechanics! Grab your metaphorical lab coat — it's about to get interesting.
2. Do Photons Even Have Size?
Now, let's consider the photon. It's a fundamental particle of light, an electromagnetic radiation carrier. Photons are massless, which already throws a wrench into our intuitive understanding of size. Something with mass has a certain spatial extent due to its physical presence. But what about something with no mass? Does it occupy space in the same way?
In the Standard Model of particle physics, photons are considered point particles. This means that, theoretically, they have no spatial extent. If you could "zoom in" on a photon (hypothetically, of course, because you can't really "zoom in" on something that's constantly moving at the speed of light), you wouldn't find any internal structure or finite size. It's just... a point.
However, that's a theoretical idealization. In reality, photons are described by their wavelength and frequency. A photon's wavelength determines its energy and color. While it doesn't define a hard "size," it does give us an idea of the spatial region over which the photon's electromagnetic field is distributed. A longer wavelength photon (like radio waves) will interact with larger objects than a shorter wavelength photon (like gamma rays).
So, in a very loose sense, you could argue that a photon with a long wavelength has a "larger" interaction area than a photon with a short wavelength. But it's not "larger" in the way a basketball is larger than a tennis ball. It's more like saying the ripple effect from a pebble dropped into a pond is "larger" than the pebble itself. The photon itself is still a point, but its influence extends over a certain area.
Electrons
3. What About the Electron's Size?
Electrons, unlike photons, do have mass. This immediately makes them seem a bit more "substantial." But even then, pinning down their size is a tricky business. Like photons, electrons are also considered point particles in the Standard Model.
This means that, theoretically, electrons also have no spatial extent. If you could hypothetically "zoom in" on an electron, you wouldn't find any internal structure or finite size. However, because electrons have charge and mass, they interact with the surrounding space, creating an electric field. This electric field can be thought of as a sort of "sphere of influence" around the electron.
The "classical electron radius" is a concept that attempts to define the size of an electron based on its charge and the energy of its electric field. It's calculated by equating the electron's rest energy to the energy stored in its electrostatic field. This gives a value of about 2.8 x 10-15 meters. However, this is just a classical calculation and doesn't accurately represent the electron's quantum mechanical nature.
Quantum mechanics describes the electron as a probability distribution, meaning it doesn't have a fixed location. Instead, it exists as a cloud of probability around the nucleus of an atom. This cloud is described by the electron's wave function, which tells us the probability of finding the electron at any given point in space. So, the "size" of an electron in an atom is really the size of this probability cloud, which can vary depending on the atom and the electron's energy level.
4. The Quantum Fuzzy Zone
The Heisenberg uncertainty principle adds another layer of complexity. This principle states that we cannot know both the position and momentum of a particle with perfect accuracy. The more precisely we know its position, the less precisely we know its momentum, and vice versa. This inherent uncertainty makes it impossible to pinpoint the exact location of an electron, further blurring the concept of its "size."
So, while electrons are considered point particles in the Standard Model, their interaction with space and their quantum mechanical behavior give them a sort of effective "size." This "size" is more about their influence on the surrounding environment than a physical dimension.
Essentially, when it comes to the quantum world, our everyday notions of size break down. It's like trying to use a hammer to tighten a screw — the tool isn't designed for the task.
We need different ways to describe and understand the properties of these fundamental particles.

Comparing Apples and Quantum Oranges
5. So, Who Wins the Size Contest?
Alright, back to the original question: Is a photon bigger than an electron? Based on the Standard Model's definition of both, technically no. Both are considered point particles, meaning they have no spatial extent. They are both infinitesimally small.
However, if we consider the "size" to be the area of influence or the extent of their interaction with the surrounding environment, the answer gets a bit more nuanced. A photon's wavelength can be used to estimate the region over which its electromagnetic field is distributed. An electron's electric field and probability distribution also give it an effective "size."
Comparing those "sizes" directly is still difficult. It's like comparing the area covered by a spotlight to the area influenced by a magnet. They're different kinds of influences, acting on different things. One is electromagnetic radiation and the other is electric field.
Ultimately, the question highlights the limitations of our classical intuition when dealing with the quantum world. The concept of "size" simply doesn't translate well to these fundamental particles. We need to think in terms of energy, momentum, charge, and other quantum properties, rather than relying on our everyday understanding of physical dimensions.

FAQ
6. Frequently Asked Questions (Because Quantum Physics is Confusing!)
Let's tackle some of the burning questions that might be swirling in your brain right now:
Q: If electrons and photons are point particles, how can atoms have size?A: Good question! The size of an atom is determined by the electron orbitals — the regions of space where electrons are most likely to be found. While the electrons themselves are point particles, their probability distribution creates a "cloud" that gives the atom its effective size. It's the arrangement and behavior of these point particles that gives rise to the macroscopic properties we observe.
Q: Does this mean everything we think we know about size is wrong?A: Not exactly! Our everyday understanding of size works perfectly well for macroscopic objects. But when we delve into the quantum realm, we need to adjust our thinking and use different models and concepts to describe the behavior of particles. Classical physics is still useful, it's just not a complete picture.
Q: So, should I just give up on understanding quantum physics?A: Absolutely not! It's challenging, yes, but also incredibly fascinating. Keep asking questions, keep exploring, and don't be afraid to embrace the weirdness. Even physicists are still trying to fully understand the mysteries of the quantum world. The journey of discovery is half the fun!

Question Video Finding The Required Wavelength Of An Absorbed Photon
